Diagnostic tests are the foundation of providing objective, critical information to healthcare providers for detection, diagnosis and prevention of disease. During the Covid-19 pandemic the need for diagnostic testing became increasingly vital to enable us to live with the virus. Many companies actively engaged in developing faster and easier ways to test people for coronavirus.
Amplification Makes It Detectable
The method underlying many molecular diagnostics is amplification – the process of encouraging the multiplication of copies of a specific viral RNA or DNA sequence in a sample, until there are so many copies that they can be detected and then measured.
Polymerase Chain Reaction (PCR), is one such method using the DNA amplification process. Traditional PCR techniques use temperature cycling to achieve amplification. Temperature cycling requires lab equipment and time. Polymerase enzymes can enable amplification without the need for temperature cycling! These enzymes allow a step-change in diagnostics as tests can be performed rapidly, in around 30 minutes outside of the lab and in elementary healthcare settings, by non-laboratory personnel at the point of care.
Making a Quality Enzyme
The critical enzymes used in the tests are made either natively from wild bacteria or by recombinant bacteria (E. coli) production methods. Both of which require cell lysis first to release the enzymes.
The choice of cell disruption method is a critical aspect of the production process to ensure:
- the highest yields of active enzymes
- easiest downstream purification
Microfluidizer® processors have been proven to achieve high levels of cell rupture and product recovery due to the ability to supply constant, controlled shear rates whilst at the same time protecting the intracellular contents. The data below shows how using a single pass through the equipment a rupture efficiency of over 90% can be achieved on lysing E. coli cells.
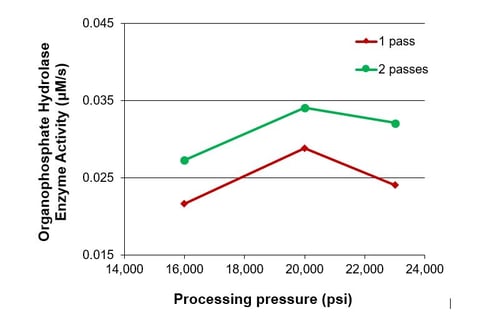

A low residence time in the processor along with only a few processing passes needed and an efficient cooling system means that the enzyme yields are high compared to other techniques available in the marketplace. Read our blog on creating quality cell disruption
Designed to be simple to use and easy to clean, Microfluidizer® processors conform to cGMP requirements. We pride ourselves on the reliability of our machines and we offer a high standard of service support and application knowledge to ensure your machine is set up to achieve your objectives.
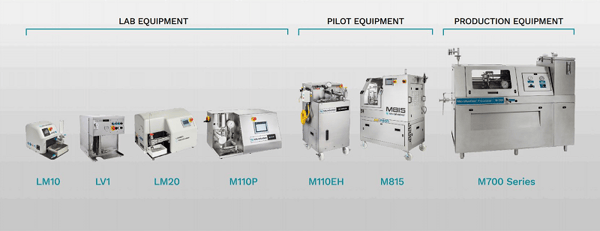
Microfluidizer® technology offers guaranteed scale-up from the lab through pilot volumes to full-scale production capabilities. Scale-up is linear, with results that are seen in the lab being replicated right up to production scale.
Rapid diagnostics already have and will continue to play a critical role in the fight against Covid-19 and many other infectious diseases, making a quality, user-friendly test is vital.
If you are working on a project, please don’t hesitate to contact us for advice and to see how a Microfluidizer® processor might enhance your process efficiency and product quality.
Topics:
Cell Disruption
