Pharmaceutical
Microfluidizer® Technology - working with
the leading pharmaceutical companies.
Microfluidizer® Processors for Pharmaceuticals
Microfluidizer® processors enable researchers to accomplish the previously impossible, such as the development of innovative methods for targeted drug delivery using multi-functional nanomedicine.
More than 1,100 pharmaceutical companies (including 19 of the top 20 companies) rely on Microfluidizer® technology as the undisputed gold standard for uniform particle size reduction for nanoemulsions, dispersions, nanoencapsulation, and cell disruption.
Customers such as Abbott Laboratories, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Schering-Plough and Wyeth all have publications acknowledging the critical contribution our pharmaceutical processors have made in developing and producing well-known drugs and vaccines on today's market.
Proven Process Efficiency
From startups to contract manufacturers to global pharma, nearly two-thirds of the more than 3,000 Microfluidizer® processors installed worldwide are used for biopharmaceutical applications. By reducing particle size uniformly, we help pharma leaders improve the quality, bioavailability, stability and process efficiency of their drugs.
RESULTS
Visible Results
Microfluidizer® processors enable researchers to develop innovative methods for targeted drug delivery using multi-functional nano-medicine.

Colitis before processing
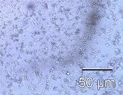
Colitis after processing

Cancer emulsion before processing

Cancer emulsion after processing
APPLICATIONS
Pharmaceutical Applications
Microfluidizer® processors are used to produce a multitude of different pharmaceutical products:
- Vaccines and vaccine adjuvants
- Injectables
- Parenteral emulsions
- Liposomes
- Antibiotics
- Inhalables
- Cancer therapeutics
- Anesthetics
- Steroids
- Drug nanosuspensions
- Ointments
ADVANTAGES
Pharmaceutical Benefits
Pharmaceutical manufacturers who use Microfluidics technology benefit from:
- 50% smaller particles than conventional homogenizers
- Improved bioavailability
- Enhanced stability for extended shelf life
- Targeted drug delivery
- Sterile filtration (< 200 nm)
- Less filter material required
- Minimized or eliminated contamination
SPECIFICATIONS
Technical Features
Microfluidizer® processors offer the following features:
- High shear particle size reduction
- Uniform particle size distribution
- Guaranteed scaleup
- Biopharma-grade models available
- Continuous crystallization
- Steam-in-Place capabilities
- Designed for cGMP/21 CFR Part 11 compliance
Pharmaceutical Manufacturing and Service Support
Pharmaceutical applications are our main focus. Microfluidics has more than 25 years of experience working with pharmaceutical companies and understands their specific requirements. In addition to manufacturing premium equipment specifically designed for high-end and complex APIs, we offer value-added services to support your success:
- Factory and on-site acceptance testing (FAT and SAT)
- Installation Qualification/Operational Qualification (IQ/OQ)
- Startup and maintenance training
- Process development consulting
- Preventive maintenance
- Scaleup consulting

