Lipid nanoparticles (LNPs) are one of the most advanced pharmaceutical delivery systems.
Used in recombinant protein and nucleic acid-based vaccines, they deliver the antigen safely and effectively, making them a key component of modern vaccine research. They increase the circulation time in the body and can help deliver the antigen to the target site.
In this blog, we provide an overview and introduction to:
- Lipid Nanoparticles, and how they affect delivery systems, and explain the benefits of LNPs as a delivery system.
- How Lipid Nanoparticles affect vaccine delivery systems
- Common preparation methods of lipid nanoparticles
- The benefits of Lipid Nanoparticles as a Delivery System
- The effect of electric charge on Lipid Nanoparticles in Vaccine efficacy
What are Lipid Nanoparticles?
With an average diameter of between 10 and 1000 nanometers, lipid nanoparticles are vesicles that include a lipid-based surfactant. For vaccine manufacturers, lipid nanoparticles allow a greater ability to customize the delivery system to determine the vaccine’s behavior in the body.
The Ways Lipid Nanoparticles Affect Delivery Systems
Here are some of the properties of lipid nanoparticles that can be adapted to aid vaccine efficacy and achieve the desired behaviors:
- Type of phospholipid
- Carrier oil (commonly cholesterol)
- Surface modifiers to increase the circulation time in the body and avoid particles being destroyed by the immune system.
- Antigen type (for example, protein or RNA)
- Processing method (e.g. thin film method using Microfluidizer® technology)
- Surface charge of the particles.
These inputs can affect the following properties of the final vaccine:
- Activity
- Immunogenicity
- Circulation time in the body
- Depot effect
- Particle size
Particle size is particularly important for lipid nanoparticles, as smaller particles allow better circulation through the body. We worked with the University of Strathclyde to discover more about the importance of vesicle size in lipid nanoparticles.
To view the webinar click here.
Techniques for Lipid Nanoparticle Formulation
The diagram below demonstrates a common technique used to produce LNPs. The phospholipid, carrier oil, and actives are dissolved in a solvent, which is then evaporated. This precipitate has a buffer added to it and is then warmed and vortexed to hydrate the phospholipids. The antigen is then added, wherein multi-layer vesicles (MLVs) are generated. This solution is processed through a Microfluidizer® processor to reduce the particle size to small unilamellar vesicles.
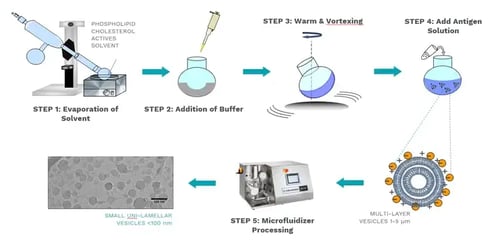
Image Ref: University of Strathclyde research
Benefits of using Lipid Nanoparticles as a Delivery System
Choosing a lipid nanoparticle delivery system yields many benefits in vaccine development. Here are just some of them:
Both hydrophilic and hydrophobic agents can be encapsulated together with high efficiency.
The lipid nanoparticles can be coated with inert and biocompatible polymers, prolonging the liposome’s circulation half-life in the body.
They can also be functionalized with specific ligands to change the way the vaccine behaves, and which specific cells, tissues, and organs it will target.
The Effect of Electric Charge on Lipid Nanoparticles in Vaccine Efficacy
Controlling the electric charge of lipid nanoparticles is a real asset in vaccine production, allowing vaccine manufacturers to dictate how the vaccine is distributed through the body. To demonstrate this, we explored the circulation rate of four different lipid nanoparticle delivery system formulations, each with different electric charges.
Anionic formulations move away from the injection site more quickly than neutral charged formulations. Conversely, cationic formulations remain at the injection site for much longer, which can form a depot effect, where the antigen is slowly released from the injection site.
For vaccine manufacturers, this depot effect can be helpful for delivery systems where a slow, steady release of antigen is desired. This can translate into fewer doses required to create immunity in the patient.
However, a depot effect can also be counterproductive if a high concentration of antigen in the blood serum is needed to cause immunogenicity.
To achieve this, the active will need to be released and circulated as quickly as possible. Negatively charged lipid nanoparticles are the best way to achieve a quick release of the antigen.
Related Reading:

