Microfluidics, was excited to host a webinar, Innovative Solvent-free Technology for Oral Bioavailability Enhancement. Our senior applications engineer, Chris Jaquin, was joined by Dr. Vanessa Bourgeaux of Skyepharma and Oksana Lemasson of LAGEPP. These speakers gave an in-depth presentation demonstrating Microfluidizer® technology's role in addressing bioavailability issues and its suitability for industrial transposition. We were pleased to have an engaged audience on the subject matter.
Below is a list of both the questions and answers from the webinar. A timestamp is provided at the end of each question so it can quickly be located on the actual webinar. (Please note: Answers may be reworded or expanded from the webinar for better clarification.)
How does this Microfluidizer® technology compare to sonication to produce nanoemulsions and scalability?
(Chris Jaquin) That's a really good question. Sonicators are typically probe-based and work by sending out very high energy sound waves in order to reduce droplet size. By their very nature, when these sound waves are sent, much of the energy will be concentrated towards the probe. However, the material at the outskirts of the container (furthest from the probe) will not be subjected to the same energy forces as the material closer to the probe. This differential can lead to issues in efficiency of nanoemulsion creation on the lab scale. When it comes to scaling, sonication companies essentially create a larger probe, which is not a directly scalable approach. Although the probe may be twice as big, it won’t necessarily give the same results if using the same formulation and simply doubling the batch size. The entire process instead would need to be re-optimized. Microfluidizer® technology differs because it simply keeps the same dimensions of micro channels and places them in parallel. By doing so, it can ensure achieving the exact same results on the production scale as achieved on the lab scale. With our technology, scalability isn't an issue whereas for some ultrasound-based technologies you will need to re-optimize your process on the production scale. (41:38)
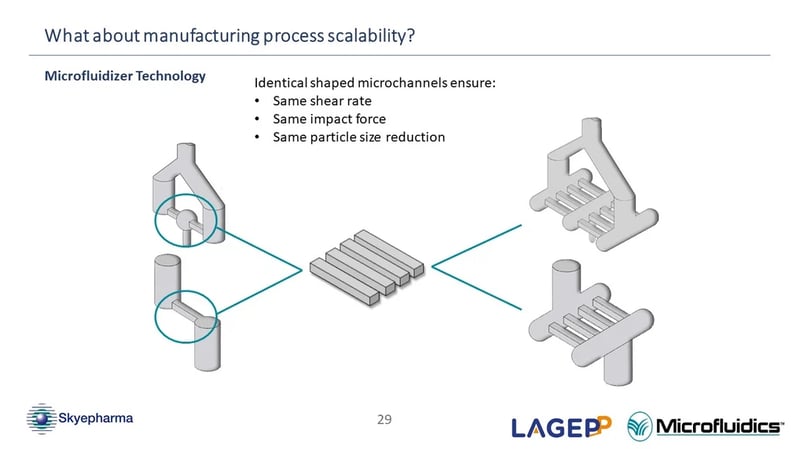
Regarding the scalability of Microfluidizer® technology, would it be a one-to-one reduction in number of passes when moving from a single channel to four channels in production, so moving from four passes to one with four channels?
(Chris Jaquin) That's a good question as well and the answer is there isn't a reduction in the number of passes that you will experience. Essentially your material isn't going to pass through all four of these micro channels, rather, the material will separate when it enters the Interaction Chamber™ so the material will pass through a single one of these micro channels. Therefore, when it comes to the number of passes, the material will experience the exact same number of passes on the lab scale as on the production scale. (43:30)
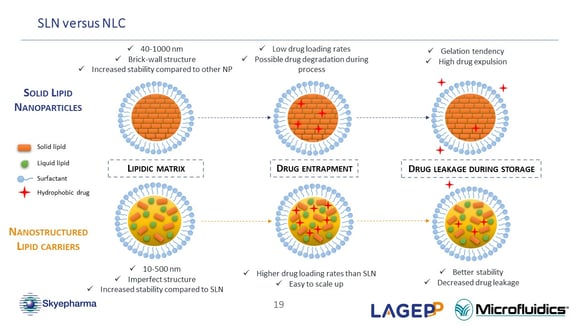
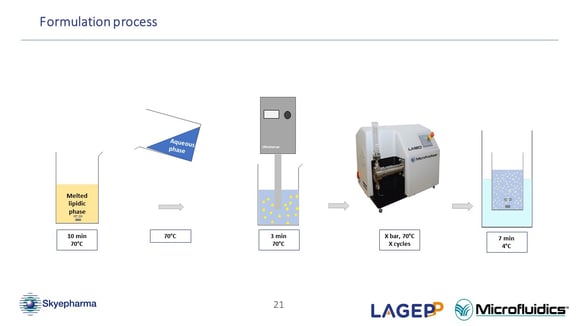
Related to NanoMicS project, could you explain a little bit more about the difference of the manufacturing process of NLC (nanostructured lipid carriers) versus SLN (solid lipid nanoparticles)?
(Oksana Lemasson) Yes, actually there's not a large difference in the process of creating these two types of lipidic nanoparticles. The only process-related difference is that for NLC (nanostructured lipid carriers), we add a liquid lipid during the emulsion preparation. More precisely, for both formulas, we have the first emulsion at the beginning of the process. The only difference is that, while melting the lipidic phase, I add the liquid lipid to the melted solid lipid. There are not many additional differences concerning the process. (44:30)
Concerning the tablet that you have manufactured, have you tried to measure the particle size by dissolving the spray dried powder or tablet?
(Oksana Lemasson) Yes. We, of course, measured it but due to confidentiality reasons, I can't divulge the exact results. What I can say is that we were able to dissolve both powder and tablets in water after the drying and tableting process. None of them showed any agglomeration issues. What is interesting is that the particle size after the dissolution of dried nanosuspension was in the same relative range of the initial nanosuspension. It suggests that the drying process does not significantly impact the nanoparticle size. (45:47)
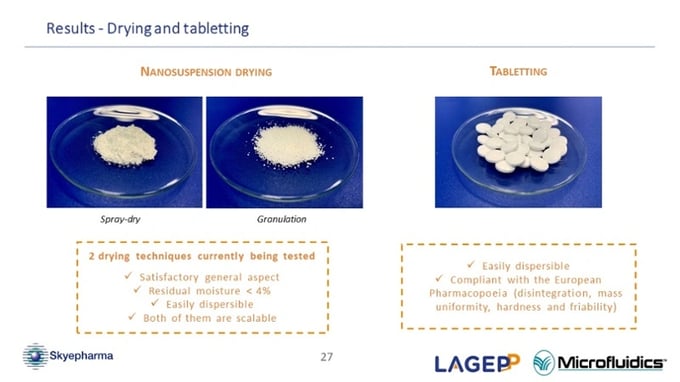
Related to the nanosuspension drying process, how is it achieved?
(Oksana Lemasson) I am currently studying two different processes. On the one hand, I tried the spray dry technique with, for the moment, classical parameters and, on the other hand, I performed a wet granulation with our nanosuspension. For both techniques, I added few excipients to enhance the drying. (46:41)
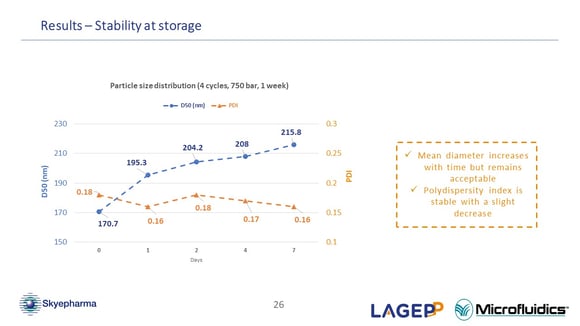
Concerning the stability of the particles that you produced, have you tried to check the particle size beyond seven days?
(Oksana Lemasson) Not for the moment, to be honest, but it is one of the topics of my PhD. It will be interesting to know after one, two or six months, how the nano suspension evolves. But for our research program, we don't really need this information for the simple reason that we are not going to wait one month between the nano suspension production and its drying. We also know that drying the nano suspension enhances its stability. (47:47)
Regarding the manufacturing process of NLC, is the cooling down of nanosuspension performed after processing through the Microfluidizer® processor or during?
(Oksana Lemasson) I do it after processing. When all the cycles are done, I collect the nanoemulsion and cool it down in an ice bath. The lipid droplets become solid, thanks to the solid lipid with low melting point, and the preparation becomes a nanosuspension. (48:58)
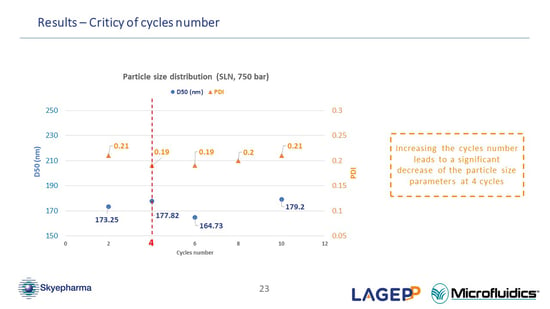
Concerning NanoMicS project, you showed a low impact of the number of cycles on the particle size and polydispersity. Why did you choose four as the optimal number of cycles compared to two, for example?
(Oksana Lemasson) That is a good question. We actually made this choice for a few reasons. After two cycles, the nanosuspension polydispersity index is slightly higher compared to the ideal feature. Secondly, after two cycles, it seems that, in our case of course, it is not sufficient to have a stable formula. The droplet size might still be too high. For our formula, the optimal and lowest number of cycles, which combines stability and good particle size parameter is four cycles. (49.19)
Last question, what is the average pressure of a Microfluidizer processor for a liter per hour?
It depends on the model. We have lab scale models with slightly lower throughputs than some of our production scale models. On the production scale models, if you need the full 30,000 psi of processing pressure, the maximum throughput you might be able to expect on one of our production scale machines would be around 5 liters per minute but on our production scale units we can actually cap them at different maximum processing pressures so we have a 10,000 psi unit, a 20,000 psi unit, and a 30,000 psi unit. If we are able to cap the maximum processing pressure at a lower pressure, 10,000 psi for example, we are actually able to get a higher throughput so it really depends on the actual model you choose and the processing pressure you select. With that said, as a maximum you might be able to expect somewhere in the range of 10 to 15 liters per minute but again we have models that are smaller pieces of equipment that can do lower throughputs as well. (50:22)
If you should have further questions, please reach out to Dr. Vanessa Bourgeaux at Skyepharma, Oksana Lemasson at LAGEPP and Chris Jaquin at Microfluidics.
Watch our entire webinar to learn more.
Posted by
Matt BaumberTopics:
Lipid Nanoparticles
